FDA Action Against Unauthorized Ozempic Products: What It Means For Patients

Table of Contents
The Dangers of Counterfeit Ozempic
Using counterfeit Ozempic, or counterfeit semaglutide in general, is incredibly dangerous. These unauthorized injections can have devastating consequences, far outweighing any potential benefits. The risks associated with fake Ozempic include:
- Incorrect Dosage: Counterfeit medications may contain significantly less or more of the active ingredient (semaglutide) than indicated. This can lead to treatment failure or serious health complications due to overdose.
- Contamination with Harmful Substances: Fake Ozempic may be contaminated with harmful bacteria, toxins, or other dangerous substances. This contamination can result in severe infections, allergic reactions, or other unpredictable health problems.
- Lack of Efficacy: Counterfeit products may contain no active ingredient at all, rendering them completely ineffective. Patients might delay proper treatment while believing they are receiving medication, potentially worsening their condition.
- Potential for Severe Allergic Reactions: Even if the counterfeit medication contains some semaglutide, the impurities and unknown ingredients can trigger severe allergic reactions, ranging from mild rashes to life-threatening anaphylaxis.
Reports of adverse events linked to counterfeit injectable medications are increasingly common. While specific numbers for Ozempic counterfeits may be limited due to underreporting, the general risk of adverse effects from any counterfeit medication is well-documented and underscores the serious threat.
FDA Crackdown on Unauthorized Ozempic Distributors
The FDA is actively working to combat the illegal distribution of unauthorized Ozempic. This crackdown involves various measures, including:
- FDA Warnings: The FDA issues public warnings and alerts about specific companies or individuals engaged in the unauthorized distribution of Ozempic and similar medications. These warnings highlight the dangers and urge consumers to avoid purchasing from these sources.
- FDA Seizures: The agency conducts seizures of counterfeit Ozempic and related products, disrupting the supply chain and preventing further distribution to patients.
- Legal Actions: The FDA takes legal action against companies and individuals found to be involved in the illegal manufacturing, distribution, or sale of unauthorized Ozempic, imposing significant fines and other penalties.
For example, [insert a link to a relevant FDA press release or news article detailing a specific case of FDA action against unauthorized Ozempic distributors if available]. These actions demonstrate the FDA's commitment to protecting patients from the risks of counterfeit medications.
Identifying Authentic Ozempic
Protecting yourself starts with knowing how to identify authentic Ozempic. Here are key steps to verify your medication:
- Check the Packaging: Carefully examine the packaging for FDA approval markings and the manufacturer's information. Look for inconsistencies or signs of tampering.
- Verify Authenticity: Use the manufacturer's website to verify the product's authenticity. Many manufacturers provide tools or methods to check if your medication is genuine.
- Purchase from Licensed Sources: Buy Ozempic only from licensed pharmacies or healthcare providers. Never purchase medication from unauthorized online retailers or individuals. Avoid suspiciously low prices, which may indicate counterfeit products.
What Patients Should Do if They Suspect Counterfeit Ozempic
If you suspect you've received counterfeit Ozempic:
- Stop Use Immediately: Do not continue using the medication. The risks significantly outweigh any potential benefits.
- Report to the FDA: Report suspected counterfeit products to the FDA immediately. You can find reporting information and contact details on the FDA website: [Insert link to the FDA MedWatch reporting site].
- Consult Your Doctor: Contact your doctor or healthcare provider to discuss alternative treatment options and assess any potential health effects from the counterfeit medication.
Protecting Yourself from Unauthorized Ozempic Products
The key takeaways are clear: counterfeit Ozempic is dangerous, the FDA is actively combating its distribution, and patients must take proactive steps to protect themselves. Always verify the authenticity of your medication and purchase only from licensed pharmacies and healthcare providers. Don't let the allure of cheaper prices put your health at risk. Protect yourself from the risks associated with unauthorized Ozempic products. Always verify the authenticity of your medication and purchase only from licensed pharmacies and healthcare providers. For more information, visit the FDA website [Insert link to relevant FDA resources].

Featured Posts
-
 Trans Australia Run On The Verge Of A New World Record
May 22, 2025
Trans Australia Run On The Verge Of A New World Record
May 22, 2025 -
 Core Weave Inc Crwv Stock Surge Reasons Behind Last Weeks Rise
May 22, 2025
Core Weave Inc Crwv Stock Surge Reasons Behind Last Weeks Rise
May 22, 2025 -
 Post Winter Pronghorn A Documentary On Community Assistance
May 22, 2025
Post Winter Pronghorn A Documentary On Community Assistance
May 22, 2025 -
 Hieu Ro Chuc Nang Hai Lo Vuong Nho Tren Cong Usb
May 22, 2025
Hieu Ro Chuc Nang Hai Lo Vuong Nho Tren Cong Usb
May 22, 2025 -
 The Goldbergs The Shows Impact On Television And Pop Culture
May 22, 2025
The Goldbergs The Shows Impact On Television And Pop Culture
May 22, 2025
Latest Posts
-
 Thong Xe Cao Toc Dong Nai Vung Tau Chuan Bi Don Lan Song Du Lich Moi
May 22, 2025
Thong Xe Cao Toc Dong Nai Vung Tau Chuan Bi Don Lan Song Du Lich Moi
May 22, 2025 -
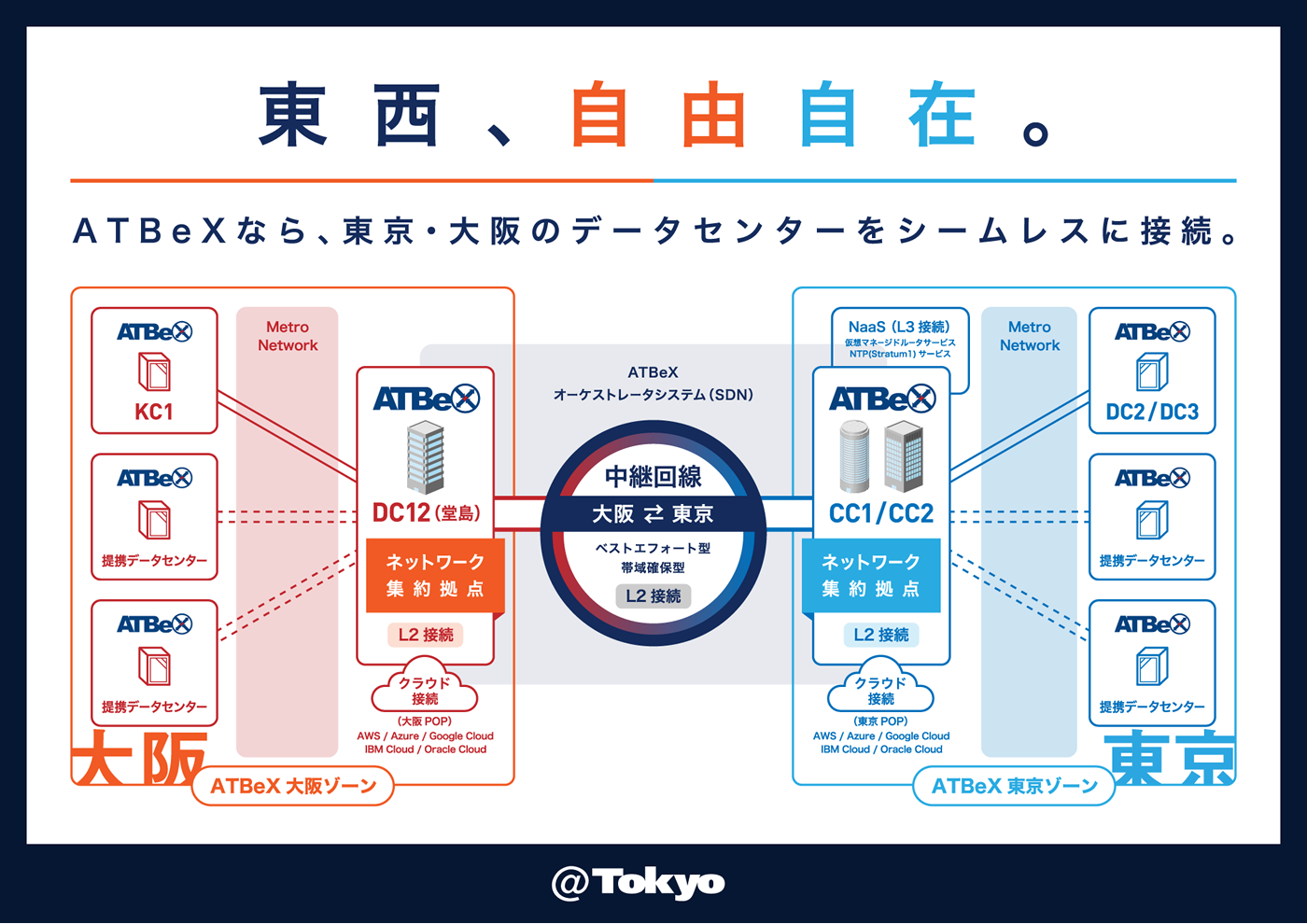 At Be X Ntt Multi Interconnect Ascii Jp
May 22, 2025
At Be X Ntt Multi Interconnect Ascii Jp
May 22, 2025 -
 Du An Cao Toc Dong Nai Vung Tau Khoi Hanh Du Kien 2 9
May 22, 2025
Du An Cao Toc Dong Nai Vung Tau Khoi Hanh Du Kien 2 9
May 22, 2025 -
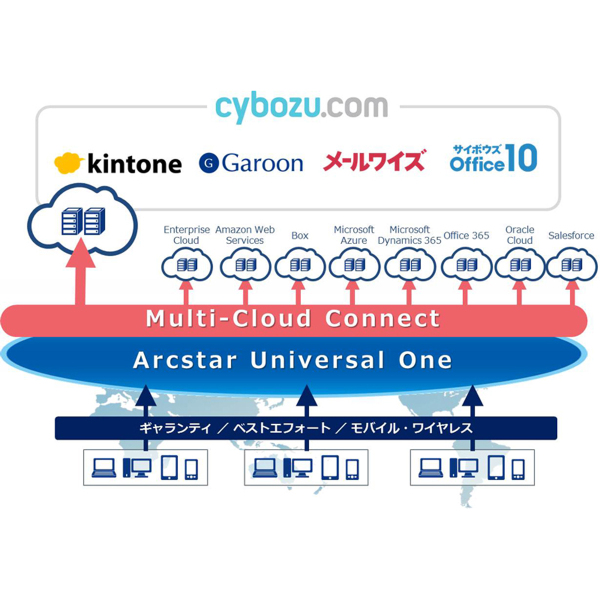 Ascii Jp Ntt Multi Interconnect At Be X
May 22, 2025
Ascii Jp Ntt Multi Interconnect At Be X
May 22, 2025 -
 Cao Toc Bien Vung Tau Dong Nai Thong Tin Moi Nhat Ve Ngay Thong Xe
May 22, 2025
Cao Toc Bien Vung Tau Dong Nai Thong Tin Moi Nhat Ve Ngay Thong Xe
May 22, 2025
