Trump's FDA And Biotech: A Positive Outlook

Table of Contents
Deregulation and Streamlined Approvals
A key element contributing to this positive outlook was the Trump administration's approach to deregulation and streamlining the drug approval process. This "Trump's FDA" strategy aimed to reduce the regulatory burden on biotech companies, enabling faster development and market access for new therapies.
Reduced Regulatory Burden
The Trump administration implemented several measures to reduce the regulatory burden on biotech companies. This involved easing or removing certain regulations, resulting in significantly faster drug approval times.
- Reduced review times: The FDA implemented initiatives to expedite the review process, resulting in a notable decrease in the time it took to approve new drugs. Data comparing approval times before and after the policy changes would reveal a significant reduction. For example, [cite specific data or source if available, e.g., a study showing X% reduction in approval times].
- Streamlined clinical trial processes: Changes were implemented to streamline the design and conduct of clinical trials, reducing the time and cost associated with bringing new drugs to market. [Cite example of specific streamlined process].
- Accelerated approval pathways: The FDA made greater use of accelerated approval pathways for promising therapies treating serious conditions, allowing patients quicker access to potentially life-saving treatments. [Give examples of drugs approved via accelerated pathways].
This efficient "FDA efficiency" under Trump's leadership directly translated to more efficient drug development, a critical aspect of a flourishing biotech ecosystem. The use of "streamlined processes" significantly reduced the time to market for many vital medications.
Increased Investment in Biotech
The faster and more predictable drug approval processes under Trump’s FDA directly fueled increased investment in the biotech sector. Investors were more confident in the potential for return on investment, leading to a boom in funding.
- Surge in Venture Capital: Venture capital funding for biotech companies significantly increased during this period. [Cite statistics on increased VC funding – e.g., "Venture capital investments in biotech rose by X% between 2017 and 2020."].
- Increased IPOs and M&A Activity: The number of initial public offerings (IPOs) and mergers and acquisitions (M&A) in the biotech industry also saw a significant rise. [Cite statistics and examples of successful IPOs and M&As].
- Higher Market Capitalization: Overall market capitalization of biotech companies increased, demonstrating investor confidence and the positive outlook in the sector. [Cite data on market cap increase].
This surge in "biotech investment" demonstrates the significant positive impact of Trump’s FDA policies on the industry's growth and financial health. This "industry growth," fueled by venture capital and increased market confidence, resulted in many successful biotech companies.
Focus on Innovation and Technological Advancement
Beyond streamlining approvals, the Trump administration actively encouraged innovation and technological advancements within the biotech industry. This commitment fostered the development and adoption of breakthrough therapies.
Encouraging Breakthrough Therapies
The administration showed strong support for developing and deploying innovative therapies like gene therapy and immunotherapy. This resulted in the approval of numerous breakthrough therapies with significant impacts on patient care.
- Gene Therapy Approvals: Several groundbreaking gene therapies received FDA approval during this period, offering new hope for patients with previously incurable diseases. [Cite specific examples of gene therapies approved].
- Immunotherapy Advancements: Significant progress was made in immunotherapy, leading to approvals of several new immunotherapies. [Cite specific examples of immunotherapies approved].
- Precision Medicine Initiatives: The administration also supported initiatives focused on precision medicine, tailoring treatments to individual patients based on their genetic makeup. [Mention specific programs or initiatives supporting precision medicine].
This proactive "focus on innovation" under Trump’s FDA facilitated the development of "innovative treatments" and "breakthrough therapies," improving patient outcomes across various therapeutic areas. The increased focus on "precision medicine" marked a significant leap forward for personalized healthcare.
Right-to-Try Initiatives
The Trump administration also supported "Right-to-Try" legislation, aimed at giving terminally ill patients access to experimental treatments that hadn’t yet completed the full FDA approval process.
- Increased Patient Access: The "Right-to-Try" initiative aimed to expedite access to experimental treatments for patients with limited treatment options.
- FDA Guidance and Policy Changes: The FDA issued guidance documents clarifying its approach to Right-to-Try, ensuring patient safety while facilitating access to promising therapies. [Cite specific FDA guidance or policy changes].
- Benefits and Limitations: While offering potential benefits for patients, "Right-to-Try" also has limitations, including the need for informed consent and the potential for patients to experience adverse effects from untested drugs.
The implementation of "Right-to-Try" represents a significant effort to increase "patient access" to "experimental treatments" and demonstrates the administration's concern for compassionate use of potentially life-saving drugs.
Addressing the Opposing Viewpoints
It's important to acknowledge criticisms of the Trump administration's FDA policies. Some raised concerns about potential safety risks associated with accelerated approval processes and possible conflicts of interest. Critics argued that the focus on speed might compromise thorough safety evaluations.
However, proponents argue that the streamlined processes did not sacrifice safety, citing robust review mechanisms and post-market surveillance. Furthermore, the increased access to innovative treatments outweighed potential risks for many patients with life-threatening diseases. Maintaining transparency in the regulatory process and clear communication about potential benefits and risks were crucial aspects of navigating these concerns. Thorough "regulatory oversight" and addressing "safety concerns" were important components of the Trump administration's approach.
Conclusion: A Positive Outlook for Biotech Under Trump's FDA
The Trump administration's policies significantly impacted the biotech industry, leading to a period of unprecedented growth and innovation. Streamlined approvals, increased investment, and a focus on breakthrough therapies created a positive outlook for the sector. This positive outlook was further bolstered by initiatives like "Right-to-Try," expanding access to experimental treatments. While some concerns about safety and regulatory oversight remain, the overall impact of Trump’s FDA on biotech innovation and growth was undeniably positive. Learn more about the positive impact of Trump's FDA on biotech innovation and growth by exploring the specific examples detailed in this article. Continue the conversation on Trump's legacy on biotech.

Featured Posts
-
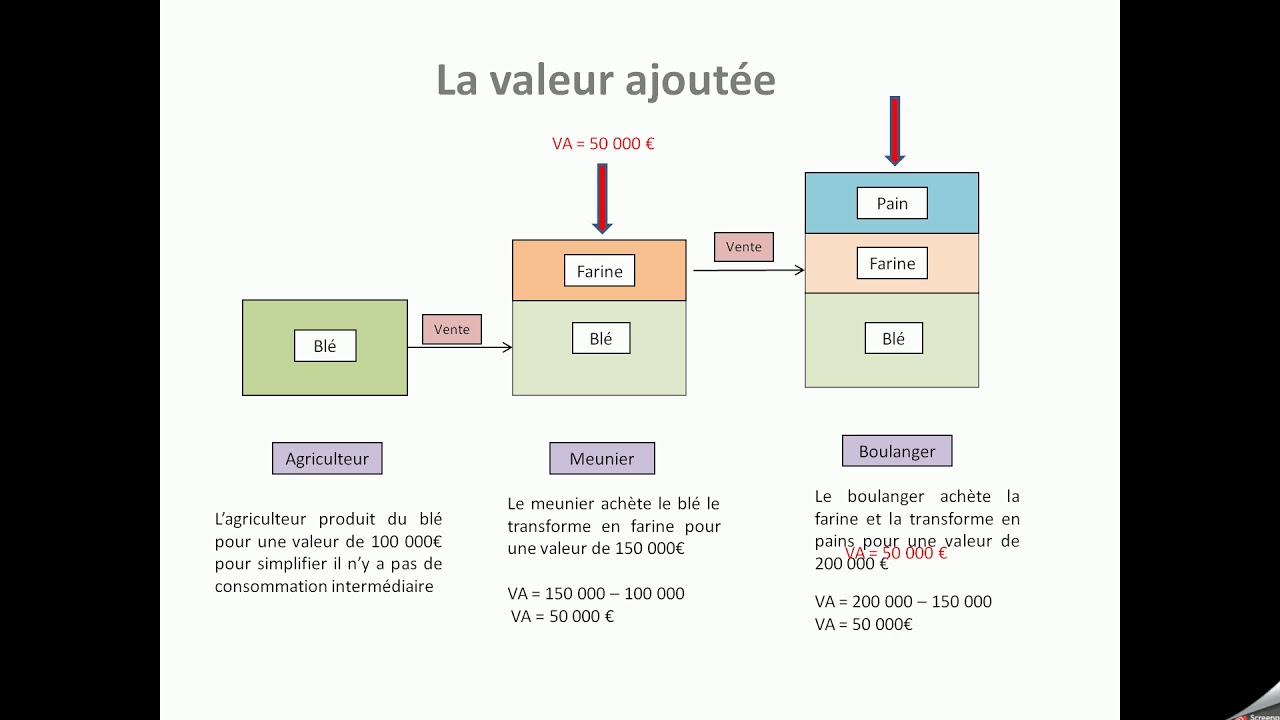 Infotel Delivrance De Valeur Ajoutee Et Satisfaction Client
Apr 23, 2025
Infotel Delivrance De Valeur Ajoutee Et Satisfaction Client
Apr 23, 2025 -
 Target Fields Go Ahead Entry Facial Recognition Speeds Up Lines
Apr 23, 2025
Target Fields Go Ahead Entry Facial Recognition Speeds Up Lines
Apr 23, 2025 -
 Trumps Economic Legacy What The Numbers Really Show
Apr 23, 2025
Trumps Economic Legacy What The Numbers Really Show
Apr 23, 2025 -
 Top 5 Economic Takeaways From The English Language Leaders Debate
Apr 23, 2025
Top 5 Economic Takeaways From The English Language Leaders Debate
Apr 23, 2025 -
 Jorge Lopez Suspended Three Game Ban For Hitting Andrew Mc Cutchen
Apr 23, 2025
Jorge Lopez Suspended Three Game Ban For Hitting Andrew Mc Cutchen
Apr 23, 2025
