FDA Crackdown On Ozempic Copies: Supply Shortages Loom
Table of Contents
The FDA's Actions and Their Impact
The FDA's recent actions against unauthorized Ozempic copies represent a significant escalation in the fight against counterfeit drugs and illegal distribution networks. These actions are aimed at protecting public health and ensuring the integrity of the pharmaceutical supply chain. The FDA's strategy involves a multi-pronged approach:
- Increased Inspections: The agency has ramped up inspections of manufacturing facilities and distribution centers suspected of producing or distributing counterfeit semaglutide products.
- Issuance of Warnings: The FDA has issued numerous warning letters to companies engaged in the illegal production and sale of unauthorized Ozempic copies. These warnings highlight the legal and health risks associated with these activities.
- Seizures of Counterfeit Products: Numerous seizures of counterfeit Ozempic and other semaglutide-based medications have been reported, disrupting the flow of illegal products into the market.
- Legal Ramifications: Manufacturers and distributors of counterfeit Ozempic copies face significant legal penalties, including hefty fines and potential criminal charges. This includes violations of FDA regulations related to the production and distribution of pharmaceuticals.
These actions, while crucial for public safety, are inadvertently impacting the legitimate supply chain. The increased scrutiny and disruption caused by the crackdown contribute to existing supply chain issues, making it harder for patients to access genuine semaglutide medications. The complexities of FDA regulations surrounding the production and distribution of medications like Ozempic contribute to these challenges.
The Growing Demand for Semaglutide and Related Medications
The soaring popularity of semaglutide-based weight-loss medications, such as Ozempic and Wegovy, has created unprecedented demand. Several factors contribute to this surge:
- Increased Awareness: Widespread media coverage and celebrity endorsements have significantly increased public awareness of these medications' effectiveness in weight management.
- Proven Efficacy: Clinical trials have demonstrated the significant weight loss achievable with semaglutide, leading to increased patient interest.
- Improved Accessibility: Increased availability through various healthcare providers has made these medications more accessible to a wider population.
This high demand, however, has strained the legitimate supply chain, leading to frequent shortages. The limited manufacturing capacity for semaglutide has struggled to keep pace with the rapidly increasing demand, creating a perfect storm for the black market to thrive. This shortage fuels the illegal production and distribution of counterfeit Ozempic copies, posing further risks to patient safety.
The Implications for Patients
The supply shortages resulting from the FDA crackdown on Ozempic copies pose significant challenges for patients relying on semaglutide medications:
- Difficulty Accessing Medication: Many patients struggle to obtain their prescribed medications due to limited availability.
- Potential for Treatment Interruption: Disruptions in medication supply can lead to treatment interruption, potentially impacting weight-loss progress and overall health.
- Health Risks Associated with Switching Medications: Patients may be tempted to switch to alternative medications without consulting their doctor, potentially leading to adverse health consequences.
- Risk of Counterfeit Products: Desperate patients might resort to purchasing counterfeit Ozempic copies from illegitimate sources, significantly increasing their risk of adverse reactions or consuming ineffective or dangerous substances.
Navigating this situation requires vigilance. Patients should only obtain medication from legitimate pharmacies and healthcare providers. Learn to identify counterfeit products by carefully examining packaging for inconsistencies or unusual markings. Always verify the authenticity of your medication with your pharmacist or doctor.
Potential Solutions and Future Outlook
Addressing the current situation requires a multifaceted approach:
- Increased Manufacturing Capacity: Pharmaceutical companies need to significantly increase their manufacturing capacity to meet the growing demand for semaglutide. Investing in advanced manufacturing technologies and expanding production facilities are crucial steps.
- Role of Pharmaceutical Companies: Pharmaceutical companies have a responsibility to proactively address supply chain challenges and ensure a consistent supply of their products.
- Development of Alternative Treatments: Research and development efforts to create alternative weight-loss medications can help alleviate pressure on the semaglutide supply.
- Enhanced Regulatory Oversight: Continued robust regulatory oversight by the FDA and other international agencies is vital to effectively combat the illegal production and distribution of counterfeit Ozempic copies and other unauthorized medications.
The ongoing efforts to combat the illegal distribution of counterfeit Ozempic copies and other unauthorized medications are crucial. A collaborative approach involving manufacturers, regulatory agencies, and healthcare providers is essential to ensure patient access to safe and effective weight-loss treatments.
Conclusion
The FDA's crackdown on unauthorized Ozempic copies, while necessary to protect public health, is exacerbating existing supply shortages and raising serious concerns about patient access. The high demand for semaglutide-based medications, coupled with the challenges of combating counterfeit drugs, necessitates a multi-pronged approach involving increased manufacturing, robust regulatory oversight, and patient education. To ensure continued access to safe and effective weight-loss medications, it is crucial to remain vigilant against counterfeit Ozempic copies and other unauthorized medications. Only purchase medications from legitimate sources to protect your health and support a stable pharmaceutical supply chain. Stay informed about FDA updates on Ozempic copies and semaglutide availability.

Featured Posts
-
 Report Manchester City Targets Arsenal Legend As Guardiolas Replacement
May 22, 2025
Report Manchester City Targets Arsenal Legend As Guardiolas Replacement
May 22, 2025 -
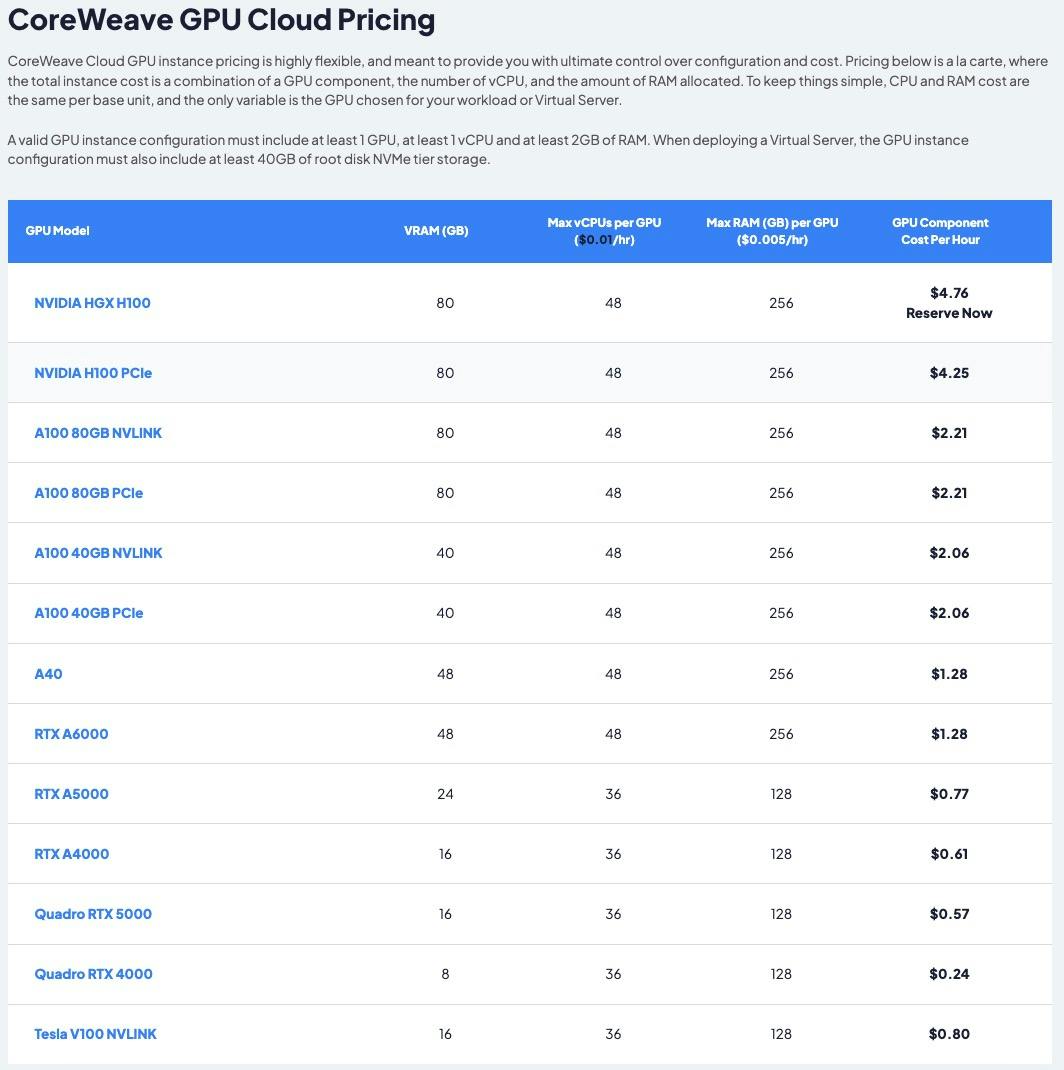 Analyzing The Current State Of Core Weave Stock
May 22, 2025
Analyzing The Current State Of Core Weave Stock
May 22, 2025 -
 Is Core Weave Crwv A Good Investment Jim Cramer Weighs In
May 22, 2025
Is Core Weave Crwv A Good Investment Jim Cramer Weighs In
May 22, 2025 -
 Dont Miss Vapors Of Morphine In Northcote
May 22, 2025
Dont Miss Vapors Of Morphine In Northcote
May 22, 2025 -
 Nato I Ukraina Poslednie Peregovory I Kommentariy Evrokomissara
May 22, 2025
Nato I Ukraina Poslednie Peregovory I Kommentariy Evrokomissara
May 22, 2025
Latest Posts
-
 Large Fire Engulfs Used Car Dealership Crews Respond
May 22, 2025
Large Fire Engulfs Used Car Dealership Crews Respond
May 22, 2025 -
 Used Car Dealership Fire Crews On Scene
May 22, 2025
Used Car Dealership Fire Crews On Scene
May 22, 2025 -
 Susquehanna Valley Storm Damage A Comprehensive Guide To Repair And Restoration
May 22, 2025
Susquehanna Valley Storm Damage A Comprehensive Guide To Repair And Restoration
May 22, 2025 -
 Susquehanna Valley Storm Damage Assessing The Impact And Recovery
May 22, 2025
Susquehanna Valley Storm Damage Assessing The Impact And Recovery
May 22, 2025 -
 Pennsylvania Thunderstorm Warning Urgent Action Needed In South Central Region
May 22, 2025
Pennsylvania Thunderstorm Warning Urgent Action Needed In South Central Region
May 22, 2025
